*Seminar Alert!* Registration is now open for our February Seminar!
*Seminar Alert!* Registration is now open for our February Seminar!
Topic: “SERENITY: developing an evidence-based decision support tool to support shared decisions about continuing or discontinuing antithrombotic medication for people with cancer towards the end-of-life” Presented by Dr. Michelle Edwards & Dr. Kathy Seddon
When: Friday 27th February 2026 [UTC 1:30 PM | CET 2:30 PM | BST 1:30 PM | EST 8:30 AM | CST 7:30 AM] Check your local time
Cost: Free
*A recording of the seminar will be made available exclusively to ISDMS members on the Society’s website approx. 1 week after the session.

We are developing a decision support tool
We are developing a decision support tool
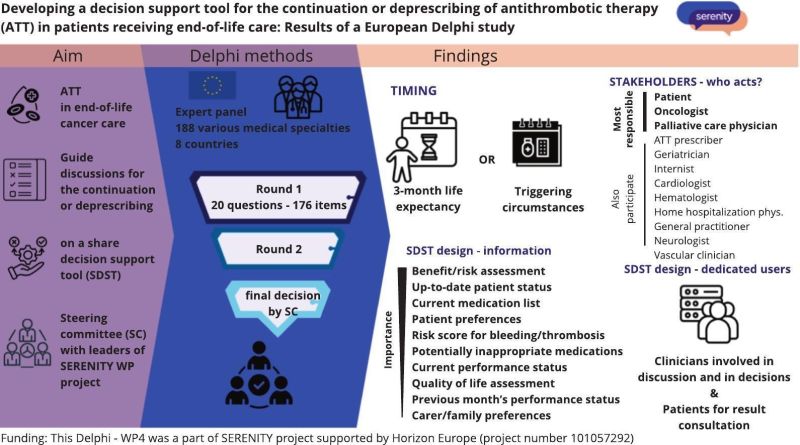
We are developing a decision support tool for the continuation or deprescribing of antithrombotic therapy in patients receiving end-of-life care: Results of a European Delphi study.
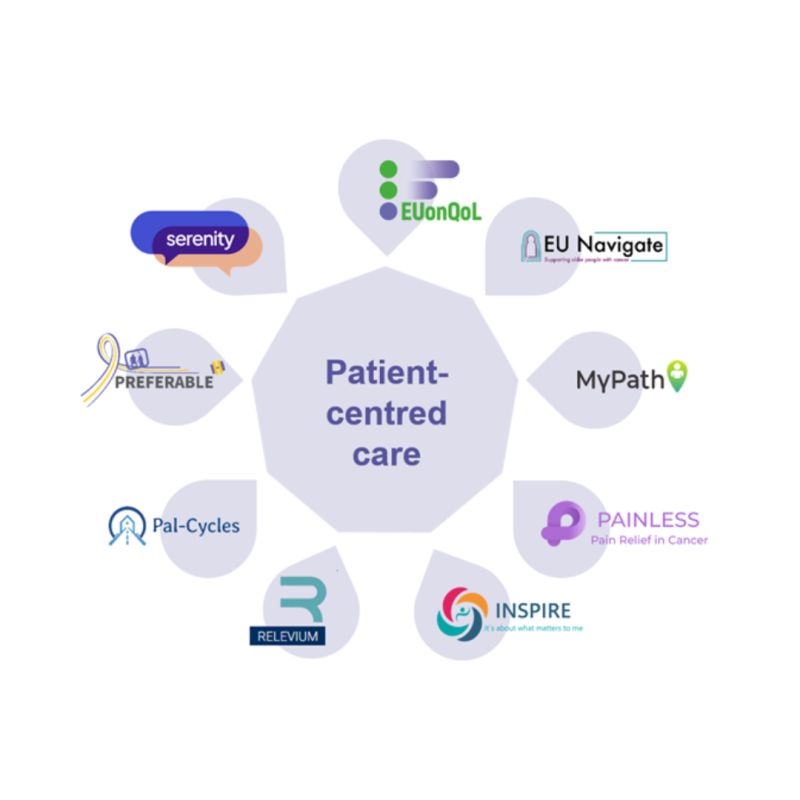
Serenity joins the Quality of Life and Patient-Centred Care (QoL-PCC) cluster
Serenity joins the Quality of Life and Patient-Centred Care (QoL-PCC) cluster
The cluster represents a key opportunity to advance oncology care. One of the main goals is to develop a unified vision around these concepts while establishing core axes related to Patient and Public Involvement (PPI) and stakeholder engagement.
Promoted by the European Commission and led by EUonQol, the cluster brings together projects such as PREFERABLE-II, EU Navigate, MyPath_EU, Pal-Cycles, INSPIRE Palliative Rehabilitation Project, RELEVIUM Project and SERENITY, all EU-funded projects working to advance palliative oncology care.
WP4: Delphi Process
WP4: Delphi Process
Through this Delphi study, the following aspects will be defined:
- characterisation of candidate patients for discussion about ATT deprescribing
- healthcare team roles in ATT decision-making
- specific information and communication requirements for patients when making deprescribing decisions
- SDST content priorities; and optimal outcomes for the planned clinical trial.
Who am I to stop taking my medication?
Who am I to stop taking my medication?
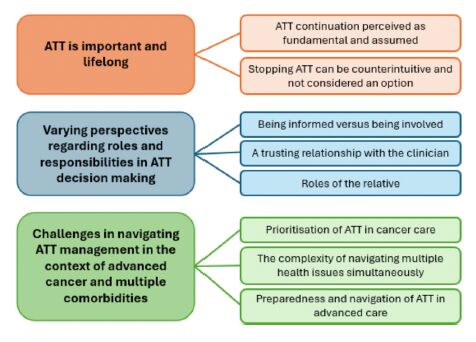
Who am I to stop taking my medication – 𝘢 𝘱𝘢𝘵𝘪𝘦𝘯𝘵 𝘢𝘴𝘬𝘦𝘥 𝘪𝘯 𝘰𝘶𝘳 𝘳𝘦𝘤𝘦𝘯𝘵 𝘴𝘵𝘶𝘥𝘺.
When we as professionals want to stop medication, we often focus on the clinical reasoning: Risk of bleeding. Limited benefit. A burden.
All true — but not enough.
Patients need more than facts. They need support to engage in that decision. And some simply don’t feel it’s their place.
This is one of the key findings in our new qualitative study, part of the SERENITY consortium:
Some patients are willing and able to co-decide about stopping antithrombotics
Others defer to clinicians, feeling it’s not their role — especially near the end of life
The difference isn’t about education or attitude. It’s about context, trust, timing, framing.
Advance care planning isn’t just about what we do — but how we decide it. Together. Stopping medication starts with the patient.
New publication in British Journal of Haematology
New publication in British Journal of Haematology
Retrospective cohort study of adults in Wales diagnosed with poor prognosis cancer between 2013 and 2021, following up patients from cancer diagnosis until death, end of follow-up or study end (31 December 2021). Outcomes included ATT discontinuation, bleeding and thromboembolic events in secondary care.
New publication about Shared decision-making
New publication about Shared decision-making
This realist synthesis explores shared decision-making (SDM) to:
(a) provide insights into why prescribing continues in end-of-life care;
(b) build a conceptual platform for optimizing ATT prescribing for persons living with cancer towards end-of-life.
Conclusion
Implementation of ATT deprescribing is enabled or constrained by
(a) the meaning of medications to patients;
(b) clinician engagement and understanding;
(c) multi-disciplinary clinical decision-making processes (including support tools);
(d) patient empowerment;
(e) organizational investment.
New publication in Thrombosis Research
New publication in Thrombosis Research
WP1: UNDERSTANDING CURRENT PRACTICE AND THE COMPLEXITY OF DECISION-MAKING IN END-OF-LIFE CARE
The study was conducted as a two-week, international, cross-sectional surveyusing flash-mob research (FMR) methodology.
The FMR steering committee invited members of the SERENITY consortium from eight European countries: Denmark, France, Germany, Italy, The Netherlands, Poland, Spain and the United Kingdom.
Our serenity consortium
Our serenity consortium
The SERENITY consortium comprises researchers and clinicians from eight European countries with specialties in different clinical fields, epidemiology and psychology.
Coordinators: Erik Klok & Professor Simon Noble
SERENITY aims to develop and evaluate a shared decision tool to support patients, their companions and healthcare professionals make evidence based and informed decisions regarding antithrombotic medicines near the end of life.
This will be achieved through a series of work packages including:
– a realist literature review
– a flash mob audit of current practice
– epidemiological database analysis of current practice in 3 countries
– qualitative interviews with patients, their companions, and clinical stakeholders
– a Delphi exercise top inform the content and design of the App
– development of a shared decision-making tool to be used as an App
– evaluation of the tool against standard practice
A targeted implementation and dissemination plan will be developed to enable the use of the SERENITY tool across Europe, as well as its incorporation in clinical guidelines and policies.
You can read the design of the study here:
Towards optimal use of antithrombotic therapy of people with cancer at the end of life: A research protocol for the development and implementation of the SERENITY shared decision support tool
New publication in HemaSphere
New publication in HemaSphere
This study evaluated vitamin K antagonist (VKA) anticoagulation control during the last year of life, using nationwide data from Statistics Netherlands, linked to anticoagulation clinics’ data and the Netherlands Cancer Registry.
Quality of vitamin K antagonist treatment during the last year of life